

MEDICALEcho Gel Pad
Medical
- Introduction of Medical Materials
- Quality Control System
- ORIGINAL PRODUCTS
- Applications
- CASE STUDIES
Addressing the problem of image acquisition of uneven and superficial parts in ultrasound diagnosis.
Echo Gel Pads do not contain scatterers and are a medium that adheres to the skin and probe while providing good delineation of body surface structures.
In ultrasound diagnosis, it is difficult to acquire images of uneven surfaces and superficial layers. By replacing conventional soft jelly with Yasojima’s Echo Gel Pad for ultrasound diagnosis, it is possible to clearly visualize irregularities and superficial areas and acquire images without artifacts.
Currently, it is used in many medical fields such as orthopedic, plastic, and oral surgeries. In addition, the demand for Yasojima’s Echo Gel Pad in industrial non-destructive inspection is growing as inspection can be done without using water.
Features and Benefits
Yasojima Proceed conducts molecular design, compounding adjustments, and molding of its Echo Gel Pad for ultrasound diagnosis in-house. We also accept consultations on the design and molding of custom-made shapes to suit the application. It is also possible to customize the hardness of the Echo Gel Pad as required.

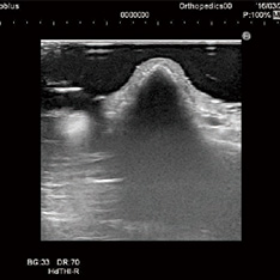
Echo Gel Pad
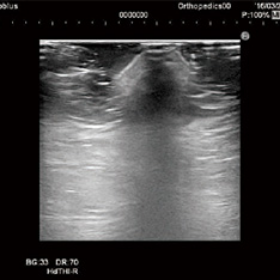
Traditional gel
Standard size
1. Echo Gel Pad
| 100 x 100 x 3mm | 1 pc | (Item number: EP-S-03) |
|---|---|---|
| 100 x 100 x 5mm | 1 pc | (Item number: EP-S-05) |
| 100 x 100 x 10mm | 1 pc | (Item number: EP-S-10) |
| 100 x 100 x 20mm | 1 pc | (Item number: EP-S-20) |
| 100 x 100 x 30mm | 1 pc | (Item number: EP-S-30) |
2. Echo Gel Pad N (for nasal bone formation)
To purchase, please contact Teijin Medical Technologies Co., Ltd
3. Echo Gel Pad F
A film is attached to one surface to improve strength and slidability.
(Please contact us for product details.)
| 100 x 50 x 3mm | 2 pc set | (Item number: EP-F-03) |
|---|
*Contact us for custom-made shapes other than those listed above.
Basic information
Material: Segmented polyurethane gel (SPUG)
| Basic physical properties measured at 20℃ | |
|---|---|
| Sound velocity | 1,389m/s |
| Density | 1,020kg/m3 |
| Acoustic impedance | 1.42×106Pa・s/m |
| Hardness (ASKER Durometer Type F) | 53 |
| Attenuation (at 5MHz) | 3.5dB/cm |
Other physical properties
Echo Gel Pad EP-S series biocompatibility
Conforms to ISO10993 “Biological safety evaluation of medical devices” (cytotoxicity, sensitization, intradermal reaction)
Disclaimer
- This is a single-use product.
- Not compatible with autoclave sterilization or EOG sterilization.
- If the product becomes discolored or cracked, discontinue use immediately.
- Please store the Echo Gel Pad according to the method described in the safety data sheet.
Document download
coming soon



















